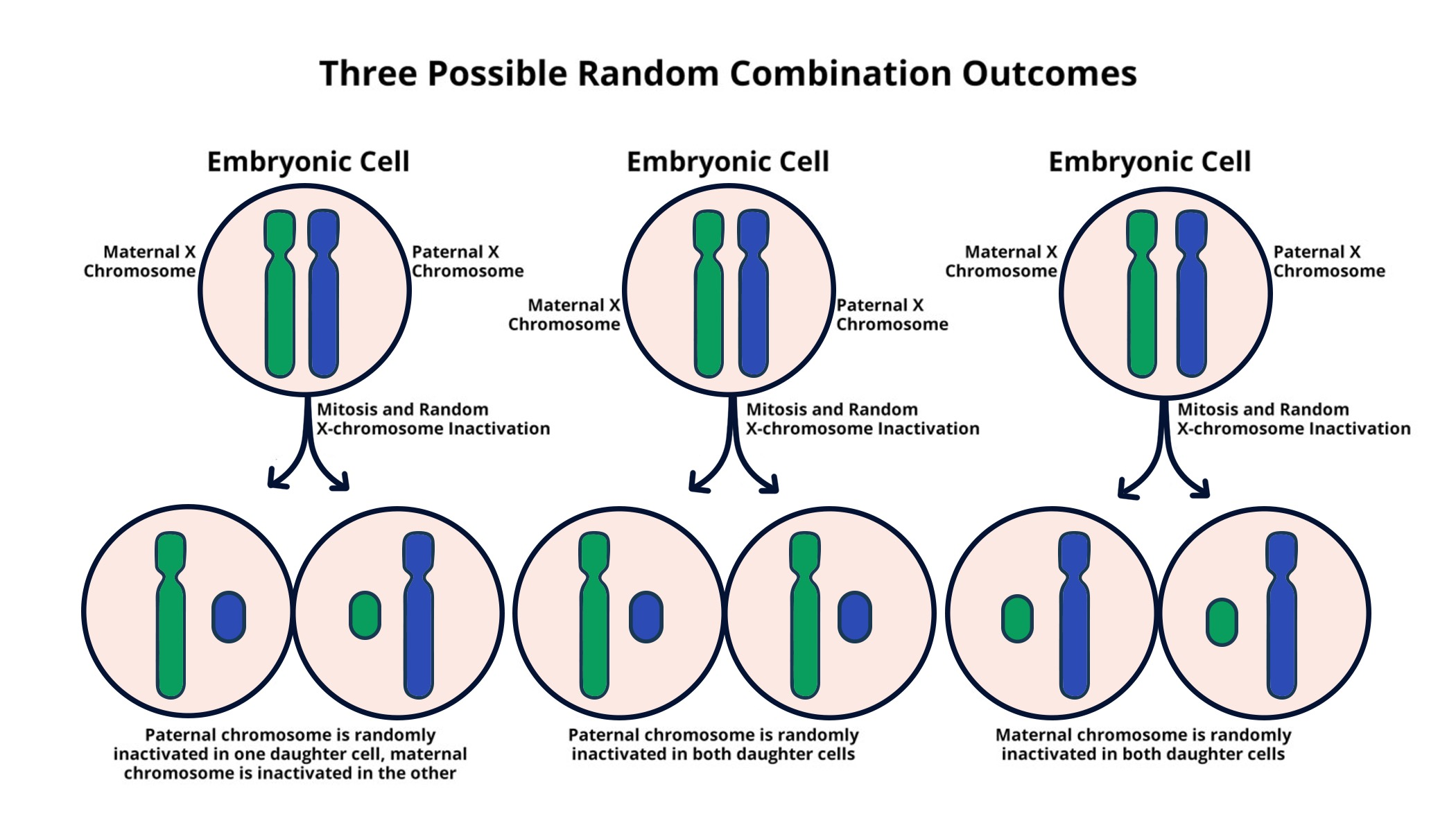
X chromosome inactivation is a fascinating biological process that plays a critical role in balancing gene expression between males and females. In females, where two copies of the X chromosome exist, one needs to be silenced to prevent gene overexpression, a phenomenon known as chromosomal silencing. This intricate mechanism not only influences normal development but is also pivotal in understanding and treating X-linked diseases, including Fragile X Syndrome and Rett syndrome. Researchers like Jeannie Lee are unraveling the complexities of X chromosome inactivation, leading to potential breakthroughs in genetic disorder therapy. As we delve deeper into this process, the promise of novel treatments for various genetic disorders becomes increasingly tangible, offering hope to countless individuals impacted by these challenging conditions.
The phenomenon of X chromosome inactivation, often referred to as lyonization, serves as a crucial mechanism that regulates gene dosage in females. This process ensures that one of the two X chromosomes in female cells remains inactive, thereby equating gene expression levels with their male counterparts, who possess a single X chromosome. Understanding this intricate process is essential, especially in the context of genetic disorders resulting from X-linked mutations. Insights gained from ongoing Rett syndrome research and Fragile X treatment studies highlight the therapeutic potential of manipulating this mechanism. As scientists explore avenues for chromosomal silencing, the prospect of innovative therapies targeting X-linked diseases becomes more promising.
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a crucial biological process that ensures dosage compensation in females, who have two X chromosomes compared to males’ single copy. Understanding how XCI works is vital, particularly as it holds the key to unlocking potential therapies for various X-linked diseases. The process involves silencing one of the X chromosomes early in embryonic development, thereby equalizing gene expression between the sexes. Researchers have long sought to uncover the molecular mechanisms that regulate this silencing, and recent studies highlight the importance of specific molecules that interact with the chromatin to facilitate this inactivation.
Recent breakthroughs, particularly from Jeannie T. Lee’s laboratory, reveal that a gelatinous substance surrounding chromosomes, likened to Jell-O, plays a significant role in the XCI process. The interaction of RNA molecules, specifically Xist, with this substance leads to structural changes that ultimately render one X chromosome inactive. By exploring these interactions in detail, scientists may uncover new avenues for treating genetic disorders, thereby offering hope to individuals affected by X-linked diseases.
The Role of Xist in Chromosomal Silencing
The molecule Xist is pivotal in the process of chromosomal silencing during X chromosome inactivation. Once expressed, Xist spreads along the X chromosome, engaging in a tug-of-war with the surrounding Jell-O-like substance. This interaction is not merely structural; it modifies the physical properties of chromatin to establish a subdued environment for gene expression. The research is particularly exciting because it suggests that manipulating Xist function could allow for selective gene activation, offering a path toward targeted therapies for conditions like Fragile X syndrome and Rett syndrome.
As the science behind Xist unfolds, the potential for therapeutic applications becomes clearer. Techniques developed by the Lee laboratory show promise in unsilencing inactivated X-linked genes, which could lead to groundbreaking treatments for patients suffering from gene mutations that impact cognition and development. This innovative research underscores the importance of genetic understanding in creating viable therapies, potentially transforming the lives of those grappling with genetic disorders.
Therapeutic Potential of Unsilencing X Chromosomes
The concept of freeing inactivated X chromosomes presents a revolutionary approach to treating genetic disorders. Traditional methods often focus on targeting symptoms rather than addressing root causes, but with advancements in understanding XCI, researchers are exploring how to reactivate genes that have been silenced due to chromosomal inactivation. For example, in diseases like Fragile X syndrome, where intellectual disability stems from mutations on the X chromosome, reactivating the healthy gene could lead to cognitive improvements.
Lesions caused by genetic disorders can restrict the function of particular genes, but unsilencing offers a pathway for rehabilitation. By leveraging techniques based on the mechanics of Xist and the surrounding chromatin, researchers aim to target specific gene mutations effectively. Current studies indicate that this approach minimizes disruption to unaffected genes, positioning these therapies as potentially safe and effective in clinical settings.
Fragile X Syndrome: A Focus of Recent Research
Fragile X syndrome, characterized by intellectual disability, represents one of the most prevalent X-linked disorders. Recent studies have focused on the relationship between chromosomal silencing and the manifestation of this condition. Research in this area seeks to decode the impact of the FMR1 gene, which mutates to cause Fragile X, and how the inactivation of its counterpart can be therapeutically targeted to ameliorate the effects of the disorder.
By understanding the mechanics of how the X chromosome is inactivated, particularly the role of Xist, scientists hope to design drugs that can effectively reactivate the FMR1 gene. Early-stage research has demonstrated the feasibility of these therapies, providing new hope for families affected by Fragile X syndrome as the medical community continues to push towards clinical trials for these revolutionary treatments.
Rett Syndrome Research Innovations
Rett syndrome, a genetic neurodevelopmental disorder primarily affecting females, has garnered significant research interest due to the potential for genetic interventions. Current research highlights the relationship between mutated MECP2 genes and the silencing processes involving X chromosome inactivation. Many researchers propose that by understanding XCI better, new strategies can be developed to counteract the effects of MECP2 mutations, offering a novel therapeutic avenue for patients.
Investigations into chromosomal silencing mechanisms, particularly by Jeannie T. Lee’s team, emphasize the potential for correcting gene function. Targeting the silenced MECP2 gene could lead to a restoration of the gene’s function, thereby alleviating symptoms associated with Rett syndrome. These insights emphasize the transformative potential of genetic research in developing targeted treatments for neurodevelopmental conditions.
Advancements in Genetic Disorders Therapy
The advancements in methods for chromosomal silencing and unsilencing herald a new era in genetic disorders therapy. As scientists decode the processes underlying X chromosome inactivation, there is great potential for breakthroughs in treating various genetic disorders that were previously viewed as untreatable. The implications extend beyond single disorders; understanding these processes could reshape therapeutic strategies across a spectrum of X-linked diseases.
A multi-faceted approach, integrating cutting-edge genetic techniques with insights from cellular biology, holds promise for addressing the underlying causes of genetic disorders instead of merely alleviating symptoms. The collaborative efforts across the scientific community could lead to significant strides in personalized medicine, allowing therapies to be tailored to individual genetic profiles, ultimately improving patient outcomes.
Chromosomal Silencing Mechanisms Explored
The mechanisms underlying chromosomal silencing are complex and multifaceted, involving an interplay between various molecular players. The research surrounding Xist, in particular, has unveiled exciting insights into how chromatin remodeling influences gene expression. Understanding these layers of regulation is crucial for deciphering how mutations can be effectively addressed in X-linked diseases.
Furthermore, chromosomal silencing extends beyond the X chromosome, suggesting that similar mechanisms may apply to other regions of the genome. This expands the relevance of ongoing research, offering potential strategies for investigating therapeutic targets across a broader array of genetic disorders. As research progresses, it is likely that findings from this field will enhance the efficacy and safety of genetic therapies.
Innovations in X-linked Disease Treatments
Innovations in treatments for X-linked diseases are becoming increasingly promising due to advancements in genetic research. The potential strategies that stem from understanding X chromosome inactivation not only aim at correcting gene function but also focus on minimizing side effects associated with genetic therapies. By designing therapeutic approaches that target inactivated genes, researchers believe they can harness the body’s cellular machinery to restore normal function in a highly specific manner.
One emerging strategy involves using oligonucleotides designed to interact with RNA in the silenced gene, effectively leading to its reactivation. As studies advance, particularly the work of Jeannie Lee’s lab, clinical applications may soon follow, positioning these therapies as groundbreaking treatments for conditions like Fragile X syndrome and Rett syndrome.
Navigating Challenges in Genetic Research
Despite the exciting prospects of genetic research into X-linked diseases, numerous challenges remain. Understanding the precise mechanisms of chromosomal silencing is crucial, yet complex, and the quest for effective therapies necessitates extensive preclinical testing to ensure safety and efficacy. Additionally, researchers must navigate ethical considerations and potential side effects that may arise from manipulating gene expression.
Moreover, while advancements are encouraging, translating laboratory results into successful clinical applications comes with its own set of hurdles. Funding, regulatory pathways, and comprehensive patient studies will all play vital roles in determining how swiftly and effectively these therapies can be brought to market. Nevertheless, as research progresses, the hope remains that solutions to these challenges can be found, ultimately leading to viable treatments for a range of genetic disorders.
Frequently Asked Questions
What is X chromosome inactivation and why is it important for understanding genetic disorders?
X chromosome inactivation (XCI) is a biological process that occurs in females, where one of the two X chromosomes is randomly inactivated to ensure dosage compensation of X-linked genes. This process is crucial for preventing diseases caused by mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome. Understanding XCI helps in developing targeted therapies for these X-linked genetic disorders by potentially unsilencing genes that are affected due to inactivation.
How does the process of X chromosome inactivation contribute to chromosomal silencing?
X chromosome inactivation involves the expression of the Xist RNA molecule, which modifies the surrounding chromatin—a gelatinous substance often referred to as ‘Jell-O’. This results in chromosomal silencing as Xist alters the material properties of the chromatin, allowing other molecules to access and inactivate the X chromosome. This regulation is essential for maintaining genetic balance in females and has implications for therapies in X-linked diseases.
Can understanding X chromosome inactivation lead to new treatments for Fragile X Syndrome?
Yes, insights from X chromosome inactivation are paving the way for innovative treatments for Fragile X Syndrome. Researchers like Jeannie Lee have discovered methods to unsilence the healthy gene trapped in the inactivated X chromosome, potentially restoring function to individuals affected by this disorder. This therapeutic approach aims to utilize the natural process of XCI to release the beneficial gene for use in cells.
What role does Xist play in the process of X chromosome inactivation?
Xist is a critical RNA molecule that initiates X chromosome inactivation. When Xist coats one of the X chromosomes, it engages with the surrounding chromatin, effectively modifying its properties to facilitate chromosomal silencing. By altering the ‘Jell-O-like’ material that surrounds the X chromosome, Xist allows for the recruitment of other factors that contribute to converting the active X genotype into an inactive X chromosome.
How might advancements in X chromosome inactivation research affect treatments for Rett Syndrome?
Advancements in X chromosome inactivation research hold promise for developing effective treatments for Rett Syndrome. By investigating how to reactivate the silenced X chromosome harboring a healthy gene, researchers aim to provide therapeutic options for those affected by this neurodevelopmental disorder. Such breakthroughs could enable the restoration of gene function, improving outcomes for patients.
What are the implications of X chromosome inactivation for males with X-linked diseases?
Although males typically have only one X chromosome and do not undergo X chromosome inactivation, they can be affected by X-linked diseases like Fragile X Syndrome. Understanding XCI may inform therapies that target the silencing of specific genes on the X chromosome in males. As researchers explore unsilencing methods, these findings could lead to therapeutic strategies that can also benefit male patients with X-linked genetic disorders.
| Key Point | Details |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes, but they must inactivate one to avoid excessive gene dosage. |
| Role of Xist | The XIST gene produces an RNA that alters the surrounding material, helping to silence the X chromosome. |
| Gelatinous Substance | The chromosomal coating, likened to Jell-O, helps in organizing chromosomes and facilitates X-inactivation. |
| Medical Implications | Research could lead to treatments for Fragile X Syndrome and Rett Syndrome by potentially unsilencing mutated genes. |
| Future Research | Ongoing optimization of therapeutic approaches and safety studies are planned toward future clinical trials. |
Summary
X chromosome inactivation is a crucial biological process that ensures females, with their two X chromosomes, do not double the dosage of X-linked gene expressions in their cells. This inactivation is mediated by specific mechanisms involving the XIST gene and a unique gelatinous substance that modifies the structure surrounding the X chromosome. The research led by Jeannie T. Lee has opened new pathways toward potential therapies for genetic disorders linked to the X chromosome. By understanding X chromosome inactivation better, researchers are optimistic about developing targeted treatments that can relieve the burden of diseases like Fragile X and Rett syndromes, keeping the focus on safety and efficacy as they move toward clinical application.